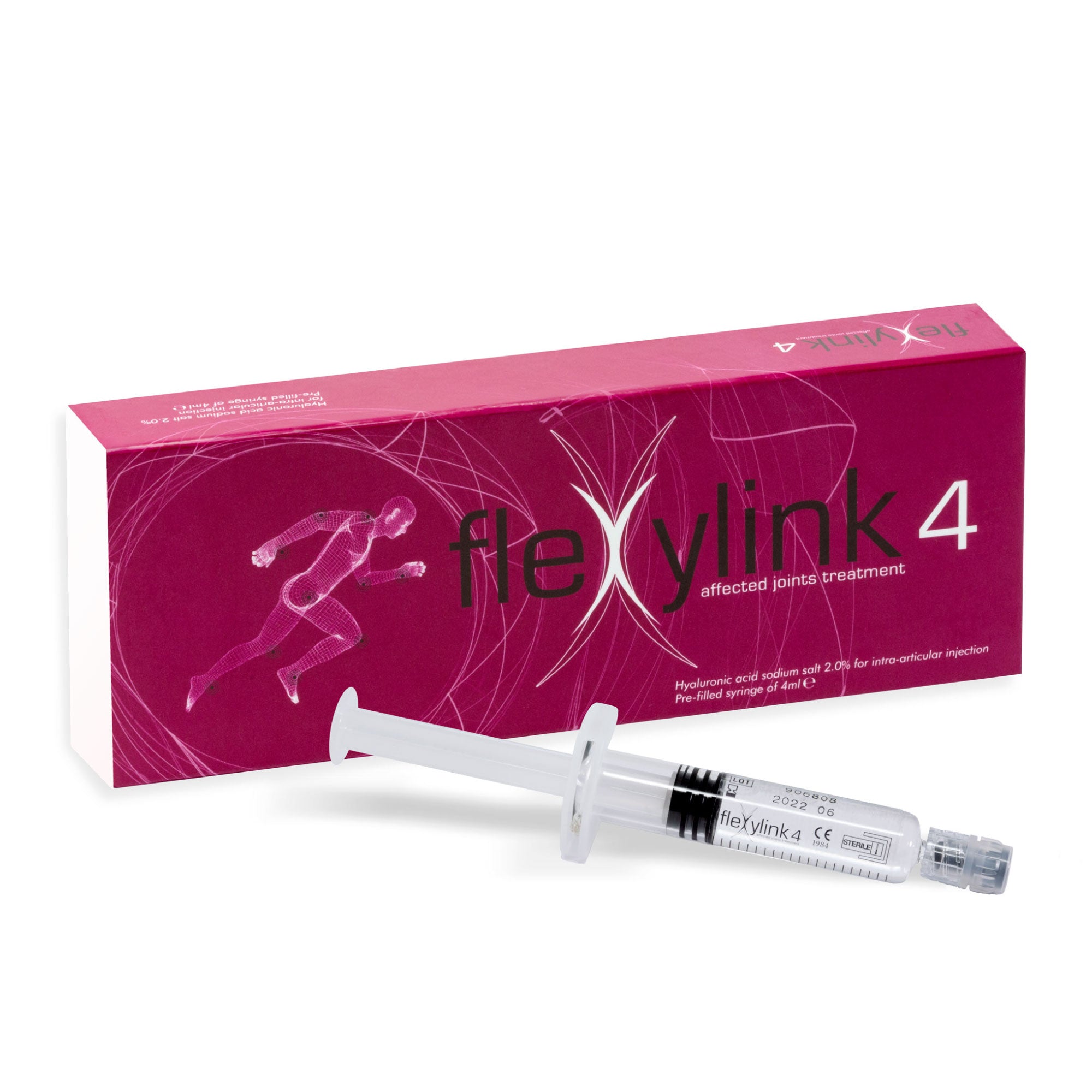
FLEXYLINK 4 - Linear Hyaluronic Acid with a High Viscosity Degree for Joints Affected by OA
 • Eccellente
• Eccellente
4 ml pre-filled syringe
TECHNOLOGICAL INNOVATION FOR OSTEOARTHROSIS THERAPY TREATMENT OF AFFECTED JOINTS
Innovative treatments based on linear hyaluronic acid with a high degree of viscosity for joints affected by OA
Flexylink 4
Linear Hyaluronic Acid 2% (HA obtained from biofermentation)
EU Classification: Medical Device Class III
HA Molecular Chain : Linear
Origin: Biofermentation (Streptococcus Equi)
Volume (pre-filled syringe): 4ml
Molecular Weight: 1500 KDa
HA Concentration: 80mg/4ml (2%)
Dosage: 2 annual therapies with 2-3 injections one every 15 days
Indications: Treatments for all joints
Packaging: Airless System (High Safety Profiles)